Years of discovery and development effort go into creating new interventions before trials and regulatory submissions get started. No matter your company size or phase of product development, regulatory submissions are challenging. Failed applications are costly, slowing approvals as the process needs to start again with longer timelines and delays in new drug availability to patients.
The process of writing submissions and quality audits (IND, INAD, NDA, NADA, etc.) involves numerous steps and requires close coordination by research, non-clinical, or clinical departments with regulatory, safety, quality, marketing, and other stakeholders. Companies can mitigate submission quality and data conformance issues in regulatory reports by automatically identifying discrepancies between information in the report and source materials. Simply put, use expert.ai to accelerate regulatory approval processes with a more accurate and comprehensive submission quality review processes.
Regulatory affairs teams focused on preclinical or clinical submissions to regulatory bodies benefit from: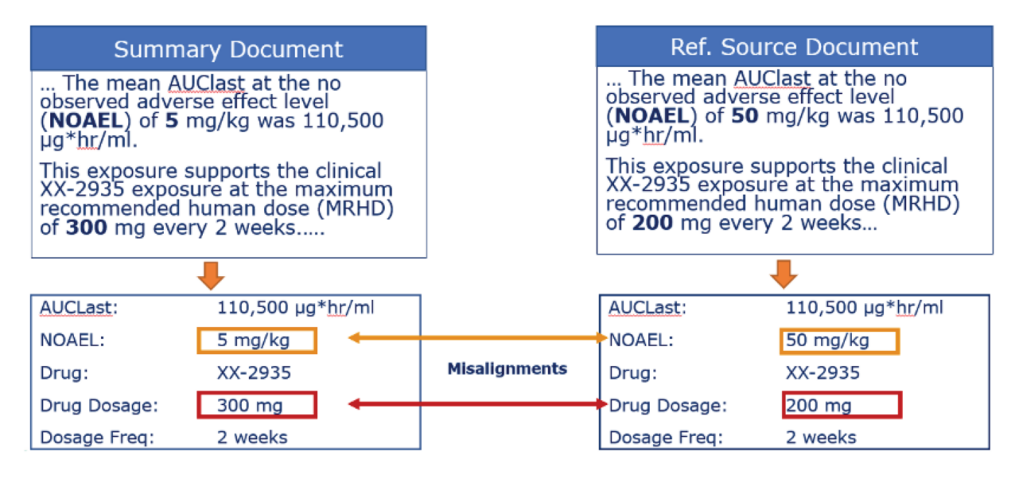
Expert.ai’s Life Sciences and Pharmaceutical solutions provide medical, research, pre-clinical, clinical, regulatory and knowledge management teams with the highest quality, standards-based and most consistent Life Sciences Knowledge Models available. Teams can now accelerate quality assurance processes for regulatory reporting by using a hybrid AI approach combining natural language processing & understanding (NLP/NLU), machine learning and Large Language Models (LLMs).
Measured Outcomes for Regulatory Teams: